Chemical and Process Engineering Resources

Designing a randomly packed column is a subtle blend of art and science. Packed columns are most frequently used to remove contaminants from a gas stream (absorption). However, packed columns can also be used to remove volatile components from a liquid stream by contacting it with an inert gas (stripping). They are also used in distillation applications where the separation is particularly difficult due to close boiling components. While we'll discuss all of these applications, we'll focus on absorption. However, the design methods are similar for any of the scenarios.
The first step in designing a packed tower is more science than art.
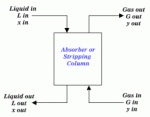 |
| Figure 1: General Packed Column Arrangement |
Since we're focusing on absorption, we'll use it as an example. In absorption/stripping, the operating line is constructed differently depending on whether the contaminated stream can be considered "dilute" or if it must be treated as a concentrated stream. Usually, it is safe to treat the stream as dilute if the contaminant makes up less than 10 mole percent of the stream. For streams that cannot be considered dilute, the mass transfer coefficients must be evaluated in terms of the gas and liquid flows. Then, graphical evaluation of several integral relationships must be completed. This type of evaluation is outside the scope of this article and a text should be consulted for solving these types of problems. For this article, we will consider dilute streams which are more common for packed tower absorption and stripping.
Dilute streams allow the column designer to assume constant mass transfer and the operating line can be constructed in terms of the simplified balance shown below:
| L out x out + G out y out = L in x in + G in y in | Eq. (1) |
Quick Example
Suppose you wish to remove acetone from a gas stream of 10,000 mol/h in a packed column. The inlet gas contains 2.6 mole percent acetone and the outlet gas stream can contain no more than 0.5 mole percent acetone. Assume a pure water stream enters the packed tower at a rate of 8,000 mol/h.
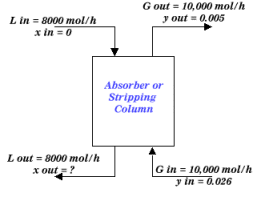 |
| Figure 2: Example Packed Column |
L out x out + G out y out = L in x in + G in y in
(8000) x out + (10000)(0.005) = (8000)(0)+(10000)(0.026)
x out = 0.02625
The equilibrium and operating lines are constructed as follows:
Just as in the McCabe-Thiele analysis of distillation, the equilibrium stages are stepped off between the two lines. Note that for stripping, the operating line would be on the other side of the equilibrium line.
Once the theoretical number of stages have been determined, you can proceed with the design of the column by following the three steps that we'll outline below.
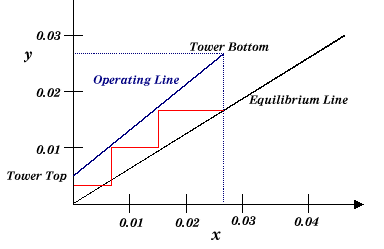 |
| Figure 3: Constructing the Operating and Equilibrium Lines |

 FB
FB

5 Comments
HAI , ANY BODY EXPLANIED ME FOR CRYOGENCIC PROCESS.
Very well explained
How to calculate number of stages in a packed absorption column.....?
if a had high flow rate of gas. i wanna absorb CO2 in MEA 30 % solution flow rate of CO2 is 9kg/s and for that flow rate MEA flow rate is 16.73 kg/s to absorb 98% CO2.Should i design packed or tray column