Chemical and Process Engineering Resources
Pervaporation, in its simplest form, is an energy efficient combination of membrane permeation and evaporation.  It's considered an attractive alternative to other separation methods for a variety of processes. For example, with the low temperatures and pressures involved in pervaporation, it often has cost and performance advantages for the separation of constant-boiling azeotropes.
Pervaporation is also used for the dehydration of organic solvents and the removal of organics from aqueous streams.ÂPervaporation can used for breaking azeotropes, dehydration of solvents and other volatile organics, organic/organic separations such as ethanol or methanol removal, and wastewater purification.
Characteristics of the pervaporation process include:
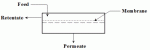 Â Â |
| Figure 1: Overview of the Pervaporation Process |
 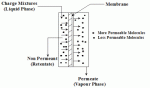 |
| Figure 2: Schematic of Liquid Permeation |
- Low energy consumption
No entrainer required, no contamination
Permeate must be volatile at operating conditions
Functions independent of vapor/liquid equilibrium
Types of Pervaporation Processes
Batch pervaporation is a simple system with great flexibility, however a buffer tank is required for batch operation. Continuous pervaporation consumes very little energy, operates best with low impurities in the feed, and is best for larger capacities.  Vapor phase permeation is preferred for direct feeds from distillation columns or for streams with dissolved solids.
Pervaporation for Separation
Liquid transport in pervaporation is described by various solution-diffusion models1. Â The steps included are the sorption of the permeate at the interface of the
solution feed and the membrane, diffusion across the membrane due to concentration gradients (rate determining steps), and finally desorption into a vapor phase at the permeate side of the membrane. The first two steps are primarily responsible for the permselectivity1. As material passes through the membrane a "swelling" effect makes the membrane more permeable, but less selective, until a point of unacceptable selectivity is reached and the membrane must be regenerated.Â
The other driving force for separation is the difference in partial pressures across the membrane. By reducing the pressure on the permeate side of the membrane, a driving force is created. Another method of inducing a partial pressure gradient is to sweep an inert gas over the permeate side of the membrane. These methods are described as vacuum and sweep gas pervaporation respectively.

 FB
FB

0 Comments