 Gas Hydrate Inhibitor Injection has been discussed previously on "Cheresources" in several posts. Besides the fact that the "Hydrate Utility" in HYSYS can calculate inhibitor injection rates, there are other methods programmable in a simple excel sheet that can be utilized to provide fairly accurate inhibitor injection rates. Equations provided by "Hammerschmidt" and "Nielsen-Bucklin" can provide good estimates for hydrate inhibitor rates and my endeavour to find alternatives to sophisticated simulation software such as HYSYS has led to the development of several excel spreadsheets related to equipment sizing and other calculations in the oil & gas field. On such effort on my part was dedicated to prepare a comprehensive calculation sheet for hydrate inhibitor injection using both the "Hammerschmidt" and "Nielsen-Bucklin" method. Since the estimation of water content in natural gas is an essential part of this calculation I had to integrate the water content calculation from the water content spreadsheet I had posted on "Cheresources" at:
Gas Hydrate Inhibitor Injection has been discussed previously on "Cheresources" in several posts. Besides the fact that the "Hydrate Utility" in HYSYS can calculate inhibitor injection rates, there are other methods programmable in a simple excel sheet that can be utilized to provide fairly accurate inhibitor injection rates. Equations provided by "Hammerschmidt" and "Nielsen-Bucklin" can provide good estimates for hydrate inhibitor rates and my endeavour to find alternatives to sophisticated simulation software such as HYSYS has led to the development of several excel spreadsheets related to equipment sizing and other calculations in the oil & gas field. On such effort on my part was dedicated to prepare a comprehensive calculation sheet for hydrate inhibitor injection using both the "Hammerschmidt" and "Nielsen-Bucklin" method. Since the estimation of water content in natural gas is an essential part of this calculation I had to integrate the water content calculation from the water content spreadsheet I had posted on "Cheresources" at:http://www.cheresour...of-natural-gas/
Let us move on to the actual background and content of hydrate inhibitor injection and the related equations
Commonly used hydrate inhibitors are Methanol & Monoethylene Glycol (MEG) for depressing the hydrate formation temperature. The "Hammerschmidt" equation gives the hydrate depression temperature as a function of the concentration (weight fraction) of the inhibitor in the final water phase & the molecular weight of the inhibitor. The actual injection flow rate is a function of the water content which condenses in the pipeline from the source pressure & temperature conditions to the destination pressure & temperature conditions. For a unboosted pipeline, this essentially means a drop in pressure & temperature.
Hammerschmidt Equation:
d = K*w / (1.8*MWinhib*(1-w))
where:
d = depression of the water dew point or gas hydrate freezing point, deg C
K = constant, dimensionless, Methanol = 2335; Monoethylene Glycol = 2700
w = mass fraction of inhibitor in final water phase
MWinhib = MW of inhibitor, Methanol = 32, Monoethylene Glycol = 62
Note:
The above equation is only applicable upto 25% by weight for Methanol & 70% for MEG in final water phase
i.e. w <= 0.25 for MeOH and w<= 0.7 for MEG
Inputs Required to set up the spreadsheet calculation:
1. Gas Flow Rate, Sm3/day
2. Upstream Gas Pressure, kPa (abs)
3. Upstream Gas Temperature, deg C
4. Downstream Gas Pressure, kPa (abs)
5. Downstream Gas Temperature, deg C
6. Hydrate formation temperature, deg C (from commercial simulation software or from the spreadsheet posted by me in my blog entry "Determining Hydrate Formation Temperature")
7. Inhibitor Used (Choose between Methanol or MEG)
8. % MEG used (Note: Methanol is generally injected as 100% by mass as Methanol whereas MEG is generally injected as water-based solution of <= 90% by mass)
Outputs obtained from Spreadsheet Calculation:
1. Upstream water Content, mg / Sm3
2. Downstream water content, mg / Sm3
3. Water Condensed, kg/day
4. "d" = Hydrate formation temperature - Downstream Gas temperature
5. "w" by solving the Hammerschmidt equation for w
6. Total qty of inhibitor = Water Condensed*w , kg/day
7. Mass rate of inhibitor in aqueous phase = Total qty of inhibitor / 1 - w, kg/day
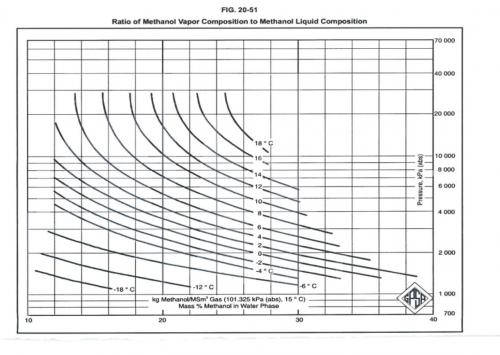
When Methanol is used as an inhibitor, vaporization losses to the gas phase need to be considered and added. The attached figure may be used to estimate vaporization losses of MeOH to gas phase.
Another important point to note is that for "d" values greater than 13.5, the "Hammerschmidt" equation using MeOH as inhibitor is not valid since the mass fraction of MeOH in water phase increases beyond 0.25. Use the "Nielsen-Bucklin" equation for MeOH concentration >0.25 mass fraction
Nielsen-Bucklin Equation:
w = xm*32.04 / (18.015 + xm*14.025)
where:
w = mass fraction of MeOH in final water phase
xm = 1 -exp(-d/72)
where:
d = depression of the water dew point or gas hydrate freezing point, deg C (>13.5)
Vaporization losses are to be calculated as explained previously.
To conclude, hydrate inhibitor injection calculations can be set up using a spreadsheet and anybody interested can do so using the guidelines provided above. I would be more than happy if somebody sets up an excel spreadsheet and requires me to check it.
Hoping to have a lot of comments from the members of "Cheresources".
Regards,.
Ankur

 FB
FB






Regards, Paulo (paulosilvajr@hotmail.com)